Prescription Drug Affordability Review Board
Colorado Prescription Drug Affordability Review Board & Advisory Council
Colorado’s Prescription Drug Affordability Board (PDAB), established under Senate Bill 21-175 and updated under House Bill 23-1225, is a Type-1 Board within the Division of Insurance that has the authority to review prescription drug costs and evaluate their impact on Coloradans through affordability reviews of prescription drugs. The Board may then recommend ways to address those costs and may set an upper payment limit for certain prescription drugs.
Open PDAB Position
We are currently accepting applications from individuals who are interested in serving a term on the PDAB.
- Individuals must have an advanced degree and experience or expertise in health care economics or clinical medicine, per statute.
- Board members are appointed by the Governor and subject to confirmation by the Colorado Senate.
Individuals who meet the requirements may apply through the Colorado Boards and Commissions website.
Open PDAAC Position
We are currently accepting applications for the following positions on the Advisory Council:
- Individuals representing healthcare consumers
- Individuals representing a labor union
- Individuals representing carriers
If you are interested in serving a 3-year term on the PDAAC, please fill out the linked application.
Listserv
To receive PDAB and PDAAC updates directly in your inbox, click here. To subscribe, you will need to enter your email and then choose Updates From Divisions > Division of Insurance > Division of Insurance Prescription Drug Affordability Board.
For questions, comments, or accessibility issues, email DOI staff at dora_ins_pdab@state.co.us.
Public Comment
To provide verbal comments to the PDAB/PDAAC, please sign up during each virtual meeting. Comments will be limited to 2 minutes per person.
To provide written comments to the PDAB/PDAAC, please email dora_ins_pdab@state.co.us up to 48 hours before each meeting.
- PDAB Members
- Dr. Sami Diab, MD
- Dr. Amy Gutierrez, PharmD
- Catherine Harshbarger, RN, MHA
- Dr. James Justin VandenBerg, PharmD, BCPS
- Board Chair - Vacant
- PDAAC Members
- Kim Bimestefer, Executive Director of HCPF
- Gail DeVore, representing health care consumers
- Open position representing health care consumers
- Dr. Bob Mulch, representing statewide health care advocacy organizations
- PDAAC Chair, Dr. Kimberley Jackson, representing health care consumers living with chronic conditions
- Open position representing labor unions
- PDAAC Vice Chair, Nathan Wilkes, representing employers
- Open position representing carriers
- Marc Reece, representing PBMs
- Dr. Richard Miranda, representing health care professionals with prescribing authority
- Dr. Brett McQueen, representing research organizations
- Katelin Lucariello, representing brand name manufacturers
- Fayez Azeez, representing generic manufacturers
- Dr. Ingrid Pan, representing pharmacists
- Leah Lindahl, representing wholesalers
- Conflicts of Interest
PDAB Conflict of Interest Policy and Procedure
PDAB Conflict of Interest Disclosure
PDAB Meetings
The PDAB meeting on February 20 has been cancelled. The next PDAB meeting will be held virtually on Friday, April 3, 2026 from 10 am - 12 pm MT.
Register for April 3 PDAB Meeting
Click here to access 2026 PDAB and PDAAC meeting links and recordings.
April 3 Meeting Materials
- Meeting agenda: coming soon.
- January 9 meeting minutes: coming soon.
- Meeting slides: coming soon.
- Written comments: coming soon.
- Draft PDAAC Recommendation Report
- 2025 Stakeholder Workgroup Comment Tracker
Note: Meeting materials from 2025 can be found under the "Public Resources" tab. Other previous materials can be accessed by emailing dora_ins_pdab@state.co.us.
PDAAC Meetings
The next PDAAB meeting will be held virtually on Thursday, March 5, 2026 from 9 - 11 am MT.
Register for March 5 PDAAC Meeting
Meeting Materials
- March 5 meeting agenda: coming soon.
- Workgroup Session 2 Meeting Slides
- Session 2 Written Comments
- PDAB Rules and Policies
- Previous PDAB Meeting Materials
Note: meeting materials not displayed on this website can be accessed by emailing dora_ins_pdab@state.co.us.
Meeting Date & Recording Meeting Materials Written Comments/Testimonies January 9, 2026 Agenda Minutes Slides Written Comments 2025 Meeting Recordings (Email dora_ins_pdab@state.co.us to receive previous meeting materials.) October 3, 2025 (Enbrel UPL Rulemaking Hearing 4) Agenda Minutes Slides Written Testimonies Written Comments September 29, 2025 Ad Hoc Agenda Minutes N/A August 22, 2025 (Enbrel UPL Rulemaking Hearing 3) Agenda Minutes Slides Written Testimonies July 11, 2025 (Enbrel UPL Rulemaking Hearing 2) Agenda Minutes Slides Written Testimonies Written Comments May 23, 2025 (Enbrel UPL Rulemaking Hearing 1) Agenda Minutes Slides Written Testimonies Written Comments April 11, 2025 Agenda Minutes Slides Written Comments February 21, 2025 Ad Hoc Agenda Minutes N/A January 17, 2025 Agenda Minutes Slides Written Comments - 2025 Upper Payment Limit Activities
The UPL rulemaking hearing for Enbrel began during the May 23, 2025 PDAB meeting. During the fourth hearing on October 3, 2025, the Board adopted the UPL rule for Enbrel and concluded the rulemaking hearing. The adopted rule can be found below.
Adopted UPL Rule for Enbrel (Etanercept)
Additional materials related to Enbrel's UPL rulemaking hearing can be found below:
- Written Testimonies
- Verbal Testimony Sign Up (currently inactive)
- Draft Proposed UPL Rule for Enbrel
- Data Submission Guide
- Confidential Information Submission Process
- UPL Benchmark Data Staff Memo
- Enbrel and Therapeutic Alternatives Benchmark Data
- Enbrel UPL Graph Packet
- Enbrel and Therapeutic Alternatives Price Data Methodology
- UPL Cost-Benefit Analysis & Regulatory Analysis
- Activities Summary Reports
- 2023/2024 Affordability Review Activities
PDAB Rulemaking
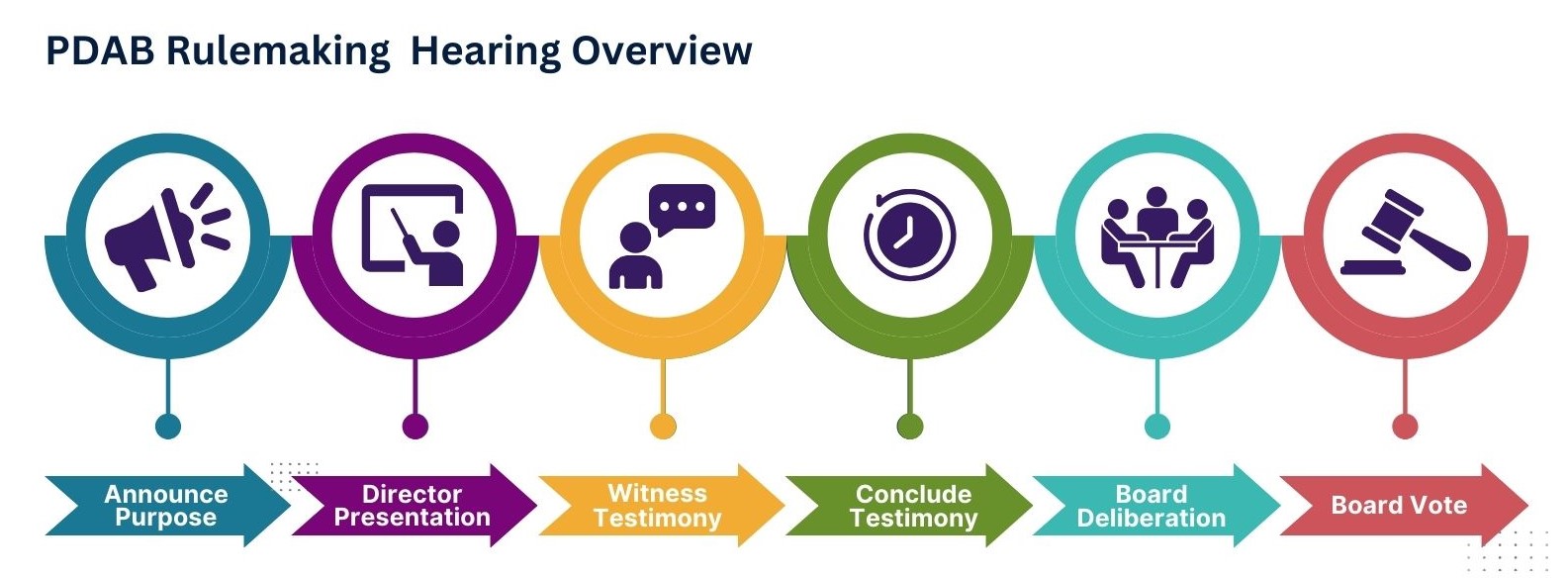
The Board is not conducting any rulemaking hearings at the time. For more information on PDAB rulemaking and how to provide testimony during a PDAB rulemaking hearing, please visit the linked PDAB Rulemaking Guide or email dora_ins_pdab@state.co.us.